what is ethanol class 10 Class edurev addition reactions ethanol substitution properties notes acid
Have you ever wondered why ethanol, a commonly used alcohol, can be converted to ethanoic acid? Well, the answer lies in the world of chemistry and specifically in the process of oxidation. When ethanol is oxidized, it undergoes a chemical reaction that results in the formation of ethanoic acid. This reaction is characterized by the loss of electrons from the ethanol molecule, which is then transferred to an oxidizing agent (such as potassium permanganate or hydrogen peroxide). One might ask, why is this important? Well, the oxidation of ethanol to ethanoic acid has numerous practical applications, including the production of vinegar, which is commonly used for cooking, cleaning and as a natural remedy for several ailments. Moreover, the conversion of ethanol to ethanoic acid is an essential step in the formation of several important chemicals, including acetic anhydride, which is commonly used in the production of plastics and textiles. To help you better understand this process, we’ve included an image from a YouTube video by Subinay Sir, a popular chemistry educator. In the image, you can see the structural formula of ethanol and its oxidation products, including ethanal (acetaldehyde) and finally, ethanoic acid.  As you can see, the oxygen atom from the oxidizing agent is added to the ethanol molecule in the form of a hydroxyl group (-OH). During this process, the hydrogen atom from the hydroxyl group is removed from the ethanol molecule and combined with another oxygen atom, forming water (H2O). This reaction is not only critical for the production of vinegar and other chemicals, but it also plays a vital role in our bodies. In fact, the oxidation of ethanol is the process by which our bodies break down and eliminate alcohol from our system. Overall, the conversion of ethanol to ethanoic acid is a significant chemical reaction with practical applications in numerous industries. Understanding this process can help us better appreciate the underlying chemistry and can help us make informed decisions about the products we use in our daily lives.
If you are searching about || 21 || Chemical Properties of Ethanol || Grade 10 || - YouTube you’ve visit to the right page. We have 5 Images about || 21 || Chemical Properties of Ethanol || Grade 10 || - YouTube like Addition Reactions, Substitution Reactions and Properties and Reactions, Properties of ethanol ||reaction of ethanol class 10||cbse X - YouTube and also Why is the conversion of ethanol to ethanoic acid an oxidation reaction. Here it is:
|| 21 || Chemical Properties Of Ethanol || Grade 10 || - YouTube
 www.youtube.comProperties Of Ethanol ||reaction Of Ethanol Class 10||cbse X - YouTube
www.youtube.comProperties Of Ethanol ||reaction Of Ethanol Class 10||cbse X - YouTube
 www.youtube.comethanol
www.youtube.comethanol
Ethyl Alcohol/Ethanol || Class 10 || Subinay Sir - YouTube
 www.youtube.comAddition Reactions, Substitution Reactions And Properties And Reactions
www.youtube.comAddition Reactions, Substitution Reactions And Properties And Reactions
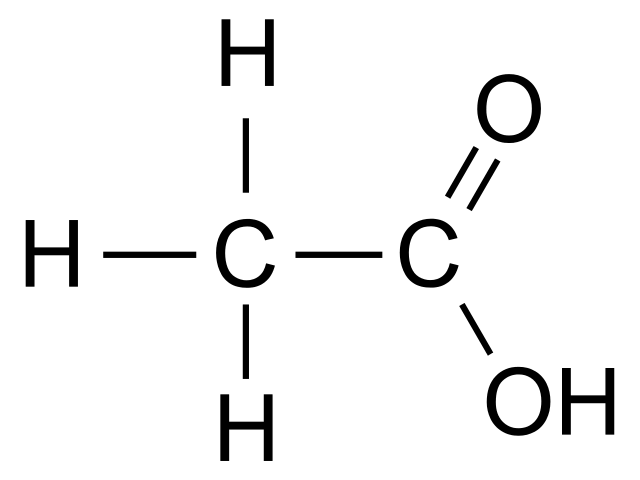 edurev.inclass edurev addition reactions ethanol substitution properties notes acid
edurev.inclass edurev addition reactions ethanol substitution properties notes acid
Why Is The Conversion Of Ethanol To Ethanoic Acid An Oxidation Reaction
www.sarthaks.comethanol ethanoic oxidation why sarthaks kmno alk acidified
Addition reactions, substitution reactions and properties and reactions. Ethyl alcohol/ethanol || class 10 || subinay sir. Why is the conversion of ethanol to ethanoic acid an oxidation reaction